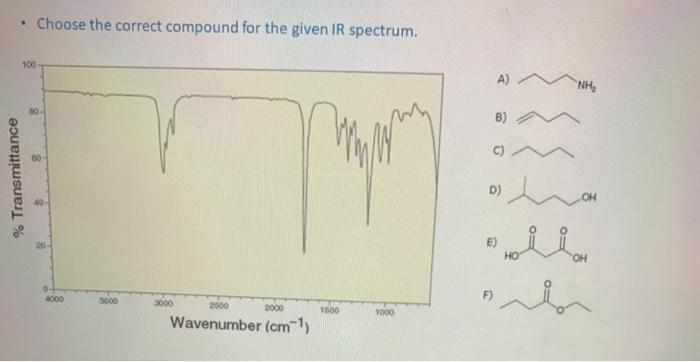
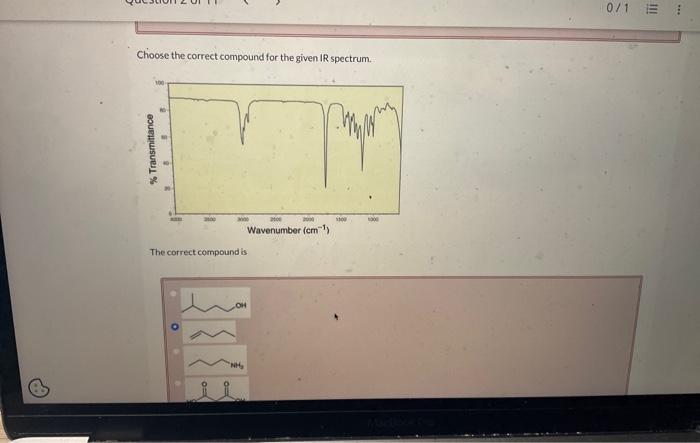
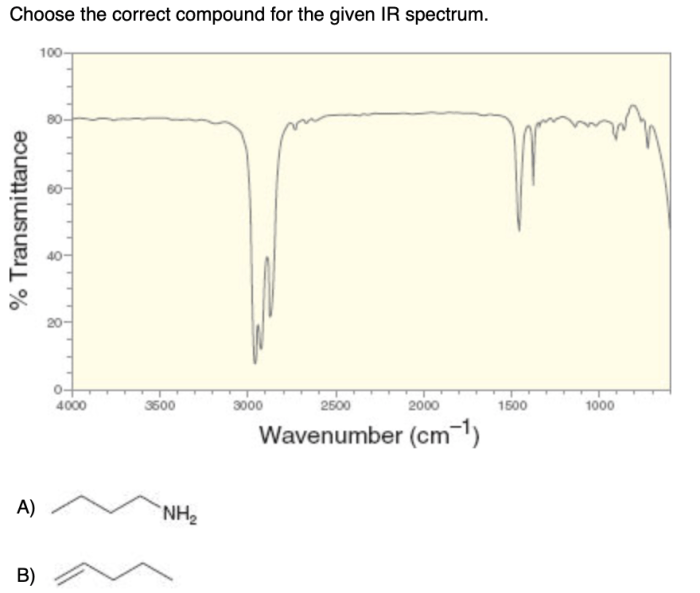
Choose the correct compound for the given ir spectrum. – Infrared (IR) spectroscopy is a powerful tool for identifying and characterizing organic compounds. By analyzing the absorption of infrared radiation by a sample, we can determine the functional groups present and, in many cases, identify the specific compound. This guide will provide a comprehensive overview of the principles and applications of IR spectroscopy, enabling you to confidently choose the correct compound for a given IR spectrum.
The fundamental principle of IR spectroscopy is that each functional group has a characteristic set of absorption bands. These bands correspond to the vibrational modes of the bonds within the functional group. By comparing the IR spectrum of an unknown compound to a library of known spectra, we can identify the functional groups present.
Introduction
IR spectroscopy is a powerful tool for compound identification. It provides information about the functional groups present in a molecule, which can be used to deduce its structure. IR spectroscopy is based on the principle that molecules absorb infrared radiation at specific frequencies, which correspond to the vibrational modes of the bonds in the molecule.
Functional Group Identification
The table below lists some common functional groups and their corresponding IR absorption bands:
| Functional Group | IR Absorption Band (cm-1) |
|---|---|
| C-H (alkane) | 2850-2960 |
| C-H (alkene) | 3000-3100 |
| C-H (alkyne) | 3300-3400 |
| C=O (ketone) | 1710-1750 |
| C=O (aldehyde) | 1720-1740 |
| C-O (alcohol) | 1050-1250 |
| N-H (amine) | 3300-3500 |
| O-H (alcohol) | 3200-3600 |
To identify functional groups in an unknown compound, compare the IR spectrum of the compound to the table above. The presence of a peak at a particular frequency indicates the presence of the corresponding functional group.
Structural Isomer Identification
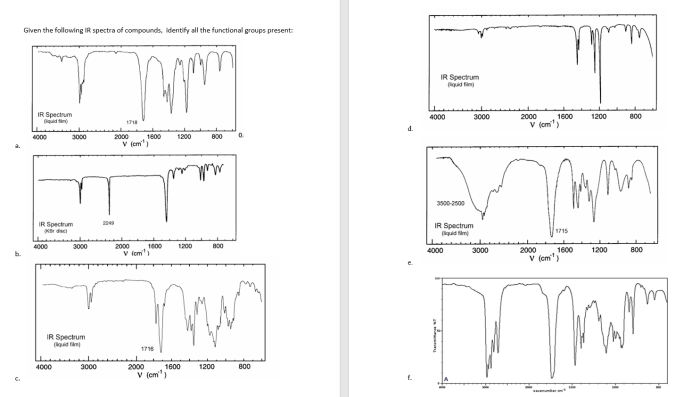
IR spectroscopy can be used to differentiate between structural isomers, which are compounds with the same molecular formula but different structural arrangements. For example, butane and isobutane are both C 4H 10, but they have different IR spectra. Butane has a strong absorption band at 2960 cm -1due to the C-H stretching vibrations of the methyl groups, while isobutane has a strong absorption band at 2870 cm -1due to the C-H stretching vibrations of the isopropyl group.
Spectral Interpretation
To interpret an IR spectrum, first identify the functional groups present in the compound. Then, look for other peaks in the spectrum that can provide information about the structure of the molecule. For example, the presence of a peak at 1600 cm -1indicates the presence of a double bond, while the presence of a peak at 1750 cm -1indicates the presence of a carbonyl group.
Applications
IR spectroscopy has a wide range of applications in different fields, including organic chemistry, biochemistry, and materials science. In organic chemistry, IR spectroscopy is used to identify functional groups and determine the structure of organic compounds. In biochemistry, IR spectroscopy is used to study the structure and function of proteins and other biomolecules.
In materials science, IR spectroscopy is used to characterize the structure and properties of materials such as polymers and ceramics.
Questions and Answers: Choose The Correct Compound For The Given Ir Spectrum.
What is the most important factor to consider when choosing a compound for IR spectroscopy?
The most important factor to consider is the presence of functional groups. Each functional group has a characteristic set of absorption bands, so it is essential to choose a compound that contains the functional groups of interest.
How can I identify the functional groups present in an unknown compound using IR spectroscopy?
To identify the functional groups present in an unknown compound, compare the IR spectrum of the compound to a library of known spectra. The absorption bands in the unknown spectrum will correspond to the functional groups present.
How can I differentiate between structural isomers using IR spectroscopy?
Structural isomers have the same molecular formula but different structural formulas. While they may have similar IR spectra, there will be subtle differences that can be used to distinguish between them. By carefully examining the absorption bands and their relative intensities, it is possible to identify the specific structural isomer.