Identify the correct values for a 2p orbital – Embark on a journey to unravel the intricacies of 2p orbitals, the cornerstone of atomic structure and chemical bonding. This guide will equip you with the knowledge and tools to accurately identify the quantum numbers that define these orbitals, enabling a deeper understanding of their properties and applications.
Delve into the fascinating world of quantum mechanics, where the behavior of electrons is governed by a set of discrete energy levels and quantized angular momentum. The 2p orbital, with its unique shape and orientation, plays a crucial role in determining the electronic structure of atoms and molecules.
1. Definition of a 2p Orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of electrons in an atom. Each orbital is associated with a set of quantum numbers that define its energy, shape, and orientation. The 2p orbital is one of the five d-orbitals in an atom.
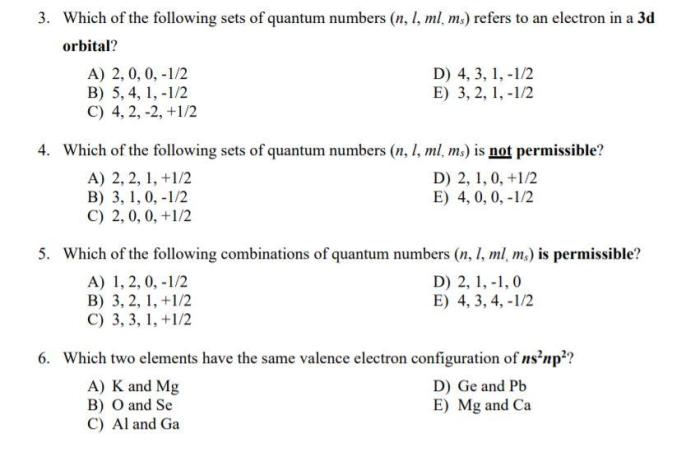
It has a dumbbell shape with two lobes oriented along the x, y, or z axis. The 2p orbital is associated with the quantum numbers n = 2, l = 1, and ml = -1, 0, or 1.
2. Properties of a 2p Orbital: Identify The Correct Values For A 2p Orbital
Energy Level, Identify the correct values for a 2p orbital
The 2p orbital has a higher energy level than the 2s orbital but lower energy than the 3s and 3p orbitals. This energy difference is due to the different shapes and orientations of the orbitals.
Magnetic Properties
The 2p orbital is paramagnetic, meaning that it has a magnetic moment. This magnetic moment is due to the electron’s spin.
Role in Chemical Bonding
The 2p orbital can participate in covalent bonding. When two atoms share a pair of electrons, the electrons occupy a molecular orbital that is formed from the overlap of the atomic orbitals. The 2p orbital can also participate in ionic bonding.
When an atom loses or gains electrons, the electrons in the 2p orbital are the most likely to be affected.
3. Identifying the Correct Values for a 2p Orbital
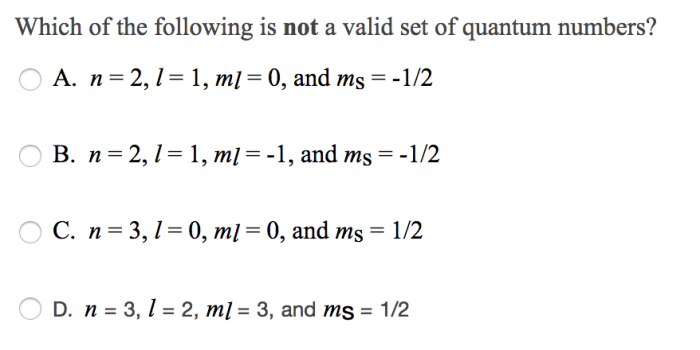
The quantum numbers for a 2p orbital are n = 2, l = 1, and ml = -1, 0, or 1. The n value indicates the energy level of the orbital, the l value indicates the shape of the orbital, and the ml value indicates the orientation of the orbital.
To identify the correct values for a 2p orbital, you can use the following steps:
- Determine the energy level of the orbital. The 2p orbital is in the second energy level.
- Determine the shape of the orbital. The 2p orbital has a dumbbell shape.
- Determine the orientation of the orbital. The 2p orbital can be oriented along the x, y, or z axis.
4. Applications of 2p Orbitals
2p orbitals play a role in various chemical reactions, including:
- Covalent bonding
- Ionic bonding
- Redox reactions
2p orbitals also contribute to the electronic structure of atoms and molecules. The number and arrangement of 2p electrons can affect the chemical properties of an atom or molecule. For example, the presence of 2p electrons can affect the electronegativity, ionization energy, and electron affinity of an atom.
Detailed FAQs
What is the shape of a 2p orbital?
A 2p orbital has a dumbbell shape with two lobes oriented along the x, y, or z axis.
How many electrons can occupy a 2p orbital?
A 2p orbital can hold a maximum of six electrons.
What is the energy level of a 2p orbital compared to other atomic orbitals?
The 2p orbital has a higher energy level than the 1s orbital but a lower energy level than the 3s and 3p orbitals.