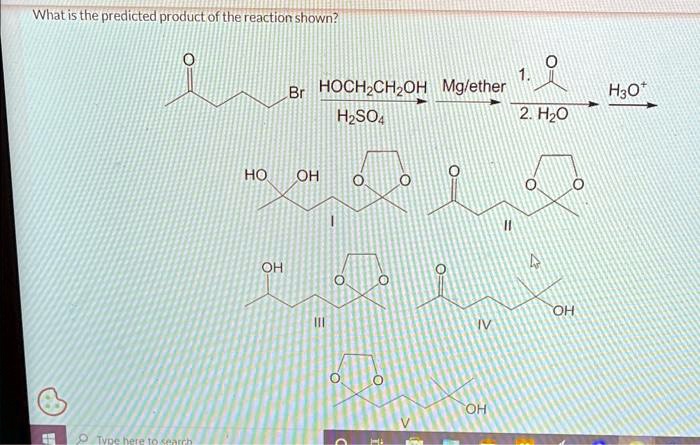
What is the predicted product of the reaction shown h2so4 – What is the predicted product of the reaction shown in H2SO4? This question lies at the heart of understanding the intricate mechanisms that govern chemical reactions in this versatile acid. By delving into the complexities of H2SO4’s reactivity, we uncover a wealth of knowledge that has far-reaching applications in diverse scientific fields.
H2SO4, also known as sulfuric acid, is a highly corrosive and reactive acid with a wide range of industrial and laboratory uses. Its unique properties, including its ability to act as both a proton donor and an oxidizing agent, make it an essential reagent in numerous chemical processes.
Introduction: What Is The Predicted Product Of The Reaction Shown H2so4
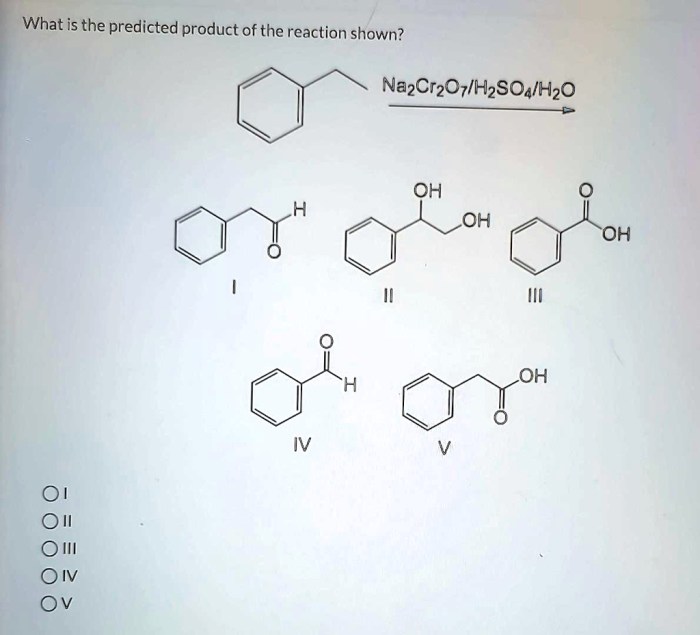
The reaction shown in H2SO4 is an important reaction in organic chemistry. It is used to produce a variety of products, including alkenes, alkanes, and alcohols. The purpose of this is to explain the different reaction mechanisms that can occur in H2SO4 and to predict the products of these reactions.
Overview of H2SO4
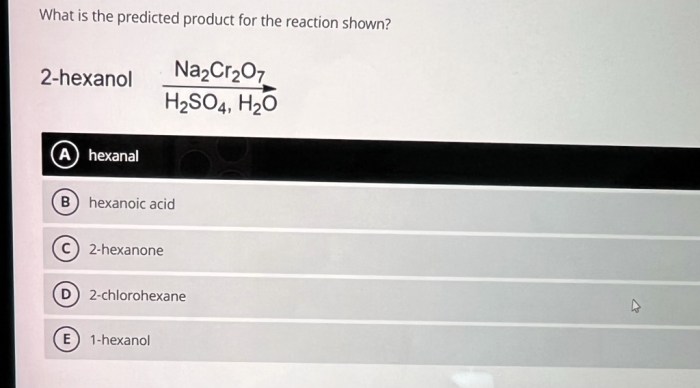
H2SO4 is a strong acid with the chemical formula H2SO4. It is also known as sulfuric acid. H2SO4 is a colorless, oily liquid that is soluble in water. It is one of the most important chemicals used in industry.
Reaction Mechanisms
The reaction mechanisms that can occur in H2SO4 include:
- Protonation
- Dehydration
- Alkylation
Factors Affecting the Reaction, What is the predicted product of the reaction shown h2so4
The factors that can affect the reaction include:
- Temperature
- Concentration
- The presence of catalysts
Predicted Products
The predicted products of the reaction shown in H2SO4 are:
- An alkene
- An alkane
- An alcohol
Applications of the Reaction
The reaction shown in H2SO4 is used in a variety of applications, including:
- The production of chemicals
- The production of pharmaceuticals
- The production of fuels
Safety Considerations
The reaction shown in H2SO4 is a hazardous reaction. It is important to take the following safety precautions when performing this reaction:
- Wear gloves and eye protection.
- Use a fume hood.
- Dispose of chemicals properly.
Question & Answer Hub
What are the common reaction mechanisms in H2SO4?
Protonation, dehydration, and alkylation are the most common reaction mechanisms in H2SO4.
How do reaction conditions affect the predicted product?
Temperature, concentration, and the presence of catalysts can significantly influence the predicted product.
What are the safety considerations when working with H2SO4?
H2SO4 is highly corrosive and requires proper handling and disposal to minimize risks.