Atomic structure worksheet answers key physical science – Embark on a captivating journey into the realm of atomic structure with our comprehensive worksheet answers key. Delving into the fundamental building blocks of matter, this guide unveils the secrets of protons, neutrons, and electrons, empowering you with a profound understanding of the microscopic world.
Our meticulously crafted explanations illuminate the intricate arrangement of electrons within energy levels and subshells, unraveling the periodic trends that govern atomic properties. Discover the profound impact of atomic structure on chemical bonding, unlocking the mysteries of ionic, covalent, and metallic bonds.
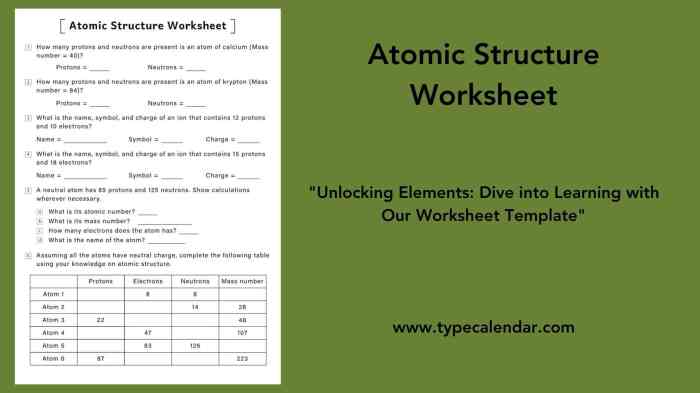
Atomic Structure
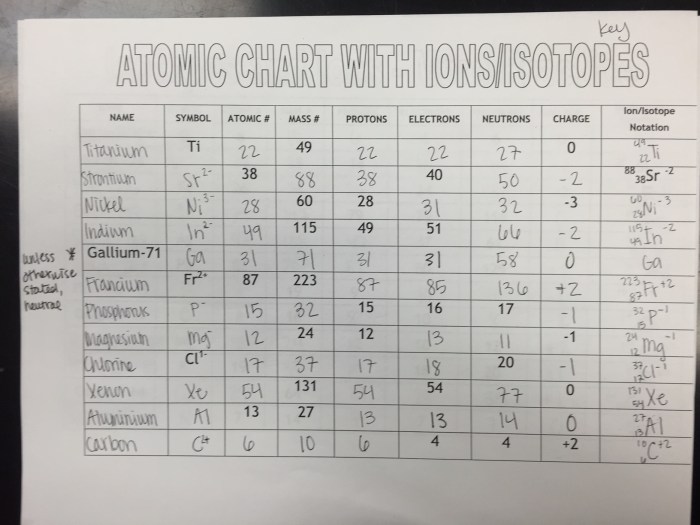
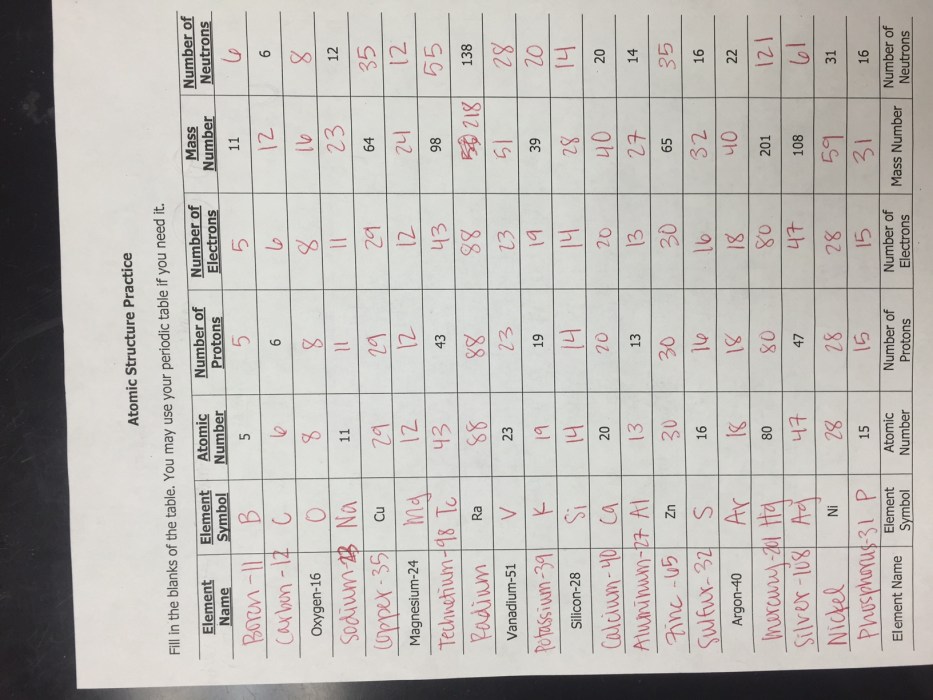
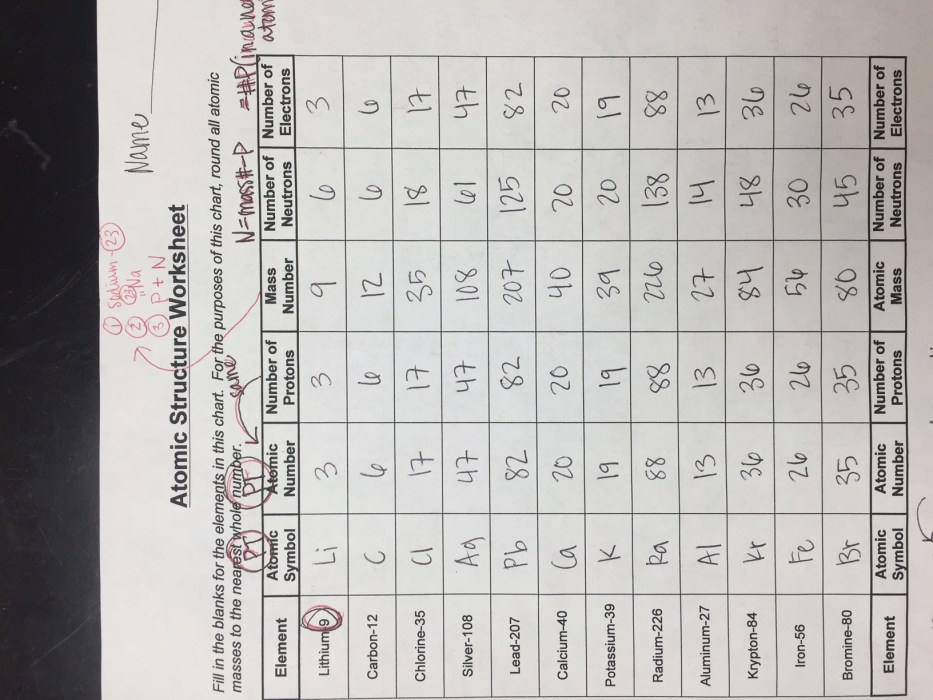
The atom is the fundamental unit of matter and the basic building block of all chemical elements. It is composed of three subatomic particles: protons, neutrons, and electrons.
Protons and neutrons are located in the nucleus, which is the central part of the atom. Protons have a positive electric charge, while neutrons have no charge. Electrons orbit the nucleus in shells, or energy levels. Each shell can hold a specific number of electrons, and the outermost shell determines the chemical properties of the atom.

Arrangement of Electrons, Atomic structure worksheet answers key physical science
Electrons are arranged in shells and subshells. The first shell can hold up to 2 electrons, the second shell can hold up to 8 electrons, and so on. Each subshell can hold a specific number of electrons, and the outermost subshell determines the chemical properties of the atom.
- s subshell:can hold up to 2 electrons
- p subshell:can hold up to 6 electrons
- d subshell:can hold up to 10 electrons
- f subshell:can hold up to 14 electrons
Key Questions Answered: Atomic Structure Worksheet Answers Key Physical Science
What is the significance of atomic structure in understanding chemical reactions?
Atomic structure provides the foundation for understanding the formation and behavior of chemical bonds, which determine the properties and reactivity of substances.
How does the arrangement of electrons in energy levels influence atomic properties?
The arrangement of electrons in energy levels determines the atomic radius, ionization energy, and electronegativity, which are key factors in predicting chemical behavior.
What are the practical applications of atomic structure in medicine?
Understanding atomic structure enables the development of targeted therapies, such as radiation therapy for cancer treatment, and the design of biomaterials for medical implants.